Introduction
There has been a rapid rise in number of patients of Diabetes mellitus in India over last few decades. Diabetic retinopathy has become a significant contributor to the load of preventable blindness in our country.
Optical coherence tomography (OCT) has revolutionised the approach to diabetic retinopathy especially associated maculopathy. It has become the cornerstone for management of diabetic macular edema and prognosticate visual outcome.
In recent literature there has been much discussion on utility of novel non-invasive biomarkers based on OCT to aid in screening patients, determining the disease severity and response to therapy. Early detection and management is the key to vision preservation.
We conducted this study to identify OCT biomarkers biomarkers that are valuable for clinical diagnosis and management of DME.
Materials and Methods
This was a prospective, observational study in the time period between April 2017 and January 2018 at Amrita Institute of Medical Sciences. Ethics and Scientific Committee approval was obtained before the start of the study. We included 37 eyes of 37 patients with treatment naïve diabetic macular edema and Hba1c less than 7. All were patients who were treated with injections of intravitreal Ranibizumab. The criteria for starting treatment with Ranibizumab injection was visual acuity less than 0.3 logMaR with centre involving diabetic macular edema >250 microns by Zeiss Cirrus HD OCT 4000.3 injections were given monthly irrespective of treatment response. After the third injection, reinjection was done only in patients who fulfilled the criteria. The sixth injection was given for all 37 patients.
All patients underwent a complete ophthalmological evaluation including best corrected visual acuity using the ETDRS vision chart, slitlamp examination, dilated fundus examination with indirect ophthalmoscopy, slitlamp biomicroscopy and OCT with the Zeiss Cirrus HD OCT 4000.Fundus Fluorescein Angiography was done in all patients before starting the treatment.
For purpose of analysis, they were divided into 2 groups-Group A had good visual outcome defined as >10 letter improvement or vision improvement to 6/6 amd Group B had poor visual outcome defined as <10 letter improvement or no improvement or worsening of visual acuity). The groups were calculated at 4 th month (after 3 injections) and at 7th month (after 6th injection.)
Optical coherence tomography analysis
The OCT scans were obtained using Cirrus Domain OCT: Zeiss. Quantitative assessment of DME included central macular thickness (CMT) that was calculated automatically by the instrument and recorded at baseline and at 4 and 7 months after the injections. Qualitative and quantitative evaluations of OCT images performed at baseline assessed the presence of several morphologic features, including (1) Subretinal Fluid(SRF) also called submacular detachment(SMD) (2) cystoid changes in the outer nuclear layer (ONL) (3) presence of cystoid changes in the inner nuclear layer (INL); (4) continuity of the inner segment-outer segment (IS-OS) layer (completely continuous, disrupted); (5) presence of Hyper Reflective Foci(HRF) and location (between the internal limiting membrane and the INL; between the outer plexiform layer and external limiting membrane; in all retinal layers); (6) status of the vitreomacular interface (attached, vitreomacular adhesion/traction); (7) presence of an epiretinal membrane(present, absent) (8) Disruption of inner retinal layers (DRIL) (present, absent) (9) The presence of intact COST line(intact, disrupted). The listed features were evaluated on 3 horizontal OCT scans: 1 encompassing the fovea, 2 scans respectively 500 mm superior and 500 mm inferior to the fovea. Grading of the OCT images was performed by 2 experienced retina specialists who were blinded to the functional and anatomic results.
Results
We studied 37 eyes of 37 patients of which 21 were males (56.8%) and 16 females (43.2%). 19 left eye s and 18 right eyes. The groups based on visual outcome were the same after third injection and sixth injection. Hence for analysis, the groups were compared after the 6th injection. Group A (good visual outcome) had 14 patients and Group B(poor visual outcome) had 23 patients. We compared the following baseline OCT characteristics between the two groups.
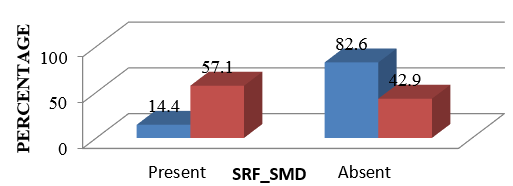
Presence of SRF/submacular detachment at baseline
Presence of SRF/SMD at baseline was found to be statistically significant between the two groups. The group with better visual outcome had 82.6% presence of SMD compared to 42.9% of those who had poor visual outcome.(p =0.012) Figure 2
Presence of cystoid spaces
Outer nuclear layer (ONL): In group A,21.4% had cystoid space in ONL compared to only 4.3% in group B but the difference was not statistically significant(p=0.1)
Inner nuclear layer (INL): In group A,21.4% had cystoid space in ONL compared to only 8.7% in group B but the difference was not statistically significant(p=0.2)
Integrity of inner segment-outersegment IS/OS junction
There was no comparable difference in the IS/OS junction characteristics between the two groups. Group A had 28.6% disrupted IS/OS junction at baseline compared to 21.7% in Group B and the difference was not statistically significant.
Presence and distribution of HRF (Hyper reflective Foci)
Presence of Hyper reflective foci (HRF) and their location (Inner layers, outer layers, all layers) were analyse between the two groups but there was no significant difference. HRF was present in 78.6% in Group A and 82.6% in group B. Distribution of HRF was also not of any statistical significance.
Vitreomacular interface abnormalities
Majority of the patients had an intact vitreomacular interface and there was no difference between the two groups. 92.9% in Group A and 78.3% in Group B had no abnormalities.
Epiretinal membrane
Both group A and B had very few patients with epiretinalmembranes and their presence had no significant diiffence in visual outcome (Only 1 eye in each group)
DRIL-Disorganisation of inner retinal layers
The presence of DRIL was comparable between the two groups at baseline-Group A had 71.4% and Group B had 71.4% patients with no DRIL at baseline and presence of DRIL at baseline did not have a significant role in visual outcome in our study. However, at 3 months and 6 months the number of patients with presence of DRIL decreased to nearly half the number from 54.1% to 24.3%.However this change was also not statistically significant.
COST line-Cone outer segment tip line
Presence of an intact COST line was also not associated with significant change in visual outcome in our study.Group A had 64.3% and Group B had 56.5% disrupted COST line and at end of 6 months this became 52,2% and 50% respectively and the difference was not significant.
Discussion
Diabetic Macular Edema (DME) is the most common cause of decrease in vision due to Diabetic retinopathy, which affects approximately 7% of all diabetic patients.1, 2
Anatomic measures on spectral-domain (SD) OCT, such as precise evaluation of individual layers, quantification of retinal thickness and macular volume, and qualitative assessment of fluid distribution and existence of hyper- reflective foci (HRF), could predict treatment success or failure to various therapies. Baseline OCT measures have been investigated regarding their predictive value in patients with DME treated by anti-VEGF therapy. It was hypothesized that distinct structural changes identifiable on SD OCT could help predict treatment responses to anti VEGF treatment, distinct from findings in eyes treated with steroid implants. The purpose of this study was to investigate whether characteristics identified on SD OCT may serve as biomarkers and predict treatment response to anti VEGF injections in patients with DME.
The pathogenesis of submacular fluid in DME remains poorly defined and it is one of the features of CSME that maybe missed in a clinical examination but is well delineated on OCT. 3 High levels of vascular leakage from the macular vasculature play a major role in its development and persistence. 4, 5 The presence of SMD does not always correlate with VA in DME.6 The predictive value of SMD at baseline for treatment response to anti-VEGF agents in DME is not clear even now with many studies supporting and refuting it. Some studies like Sophie et al and Fickweiler et al have reported significant improvement in VA when SRF was present at baseline.7, 8 However, many others like Shimura et al and Seo et al have found no difference or even an association with worse functional results. 9, 10 In the RISE and RIDE studies, when an post hoc analysis was done, the presence of submacular fluid predicted excellent visual outcomes in patients treated with ranibizumab. 7
In our study also, presence of SRF was the single most important biomarker in predicting good visual acuity response to anti VEGF injections.
In a study by Dinah Zur et al, for the International Retina Group, they studied 299 patients treated with dexamethasone implants and found presence of SRF, absence of Hyperreflective Foci(HRF) and intact IS/OS junction to be predictors of good visual outcome.3 However in our study, we found a statistically significant outcome only in the presence of SRF and absence of HRF or intact IS OS were not statistically significant.
In DME, cystoid spaces can be related to specific intraretinal layers on SD OCT. The inner and outer plexiform layers may present physical resistance to fluid movements and changes in DME are cannot be limited to one layer and involve several layers at the same time. 11, 12 In our study, 89.2% of all patients presented with ONL cysts; of them, the majority had large ONL cysts (>200 mm), which mostly occur at a relatively late stage of the disease. Previous studies reported that large ONL cysts predict worse VA outcome after anti-VEGF treatment. 13, 14 this maybe because Cystoid changes beneath the outer plexiform layer have been shown to present risk to photoreceptor cells and iimpair IS-OS integrity leading to an adverse effect on central visual functions. 15 In our study, the location of intraretinal cysts did not alter functional outcomes with no significant difference between the two groups. However, the size could not be separately analysed because of the small sample size. This is similar to Dinah Zur et al study on Dexamethasone implants.3 It is postulated that more than the absolute cyst size and volume, the remaining tissue between cysts in the central macula seems to be crucial for good visual acuity.16 We did not analyse this parameter, and further investigation might provide more information about the significance of this.
In our study, majority with good visual outcome had no vitreoretinal interface abnormalities or epiretinal membrane (92.9%) but 7.1% did have which supports a subanalysis of the Ranibizumab for Edema of the Macula in Diabetes trial, which showed that the presence of vitreomacular adhesion itself does not preclude worse functional outcome in eyes with DME treated with ranibizumab. 17
Hyperreflective foci (HRF) signifies lipoprotein extravasation after breakdown of the inner blooderetinal barrier in the initial stages of the development of intraretinal hard exudates. 18 The predictive value of HRF on visual outcome after anti-VEGF treatment in DME is not very clear. 19 In our study, the majority of eyes presented with HRF at baseline (81%) but neither its presence nor location were predictors of good visual outcome.
DRIL is the failure to identify any of the boundaries of the ganglion cell layer-inner plexiform layer (GCL-IPL) complex, INL, and OPL. DRIL is assessed independently of intraretinal cysts, epiretinal membrane, subretinal fluid, or any other OCT-evident pathology. Intraretinal cysts are commonly seen in the outer nuclear layer, resulting in overall retinal thickening; however, if the inner retinal layers can still be demarcated, then DRIL is not considered present. In addition, loss of the normal foveal contour does not constitute DRIL by itself unless there is concurrent loss of retinal layer boundaries.
Absence of DRIL at baseline and decrease in its extent during treatment was first shown to correlate with better visual acuity gain by Sun et al 20 but in our study, although 50% eyes regained inner retinal morphology(DRIL was decreased) the difference was not predictive of good outcomes. Similarly, COST line disruption also did not show any significant difference between the two groups. One reason for this could be that we have not evaluated the extent of the DRIL or disruption of COST line in microns, only the presence or absence was taken into consideration. This and the smaller sample size could account for the lack of statistical significance.
Conclusion
Our study evaluated various qualitative OCT biomarkers for predicting good visual outcome in diabetic macular edema treated with Ranibizumab. Presence of SRF/SMD was found to have most significance in predicting good visual acuity response to anti VEGF. DRIL was found to decrease during the treatment but a predictive role could not be demonstrated. We are limited by the smaller sample size and a larger study would throw more light on this investigative modality as a predictor of visual outcomes in diabetic macular edema.