- Visibility 299 Views
- Downloads 10 Downloads
- DOI 10.18231/j.ijooo.2022.022
-
CrossMark
- Citation
Recent update on pterygium
- Author Details:
-
Ashish Gupta
-
Rajendra Prakash Maurya *
-
Manisha
-
Syeed Mehbub Ul Kadir
-
Amit Patel
-
Asha Devi
-
Eshwari Patel
-
Shivangi Singh
Introduction
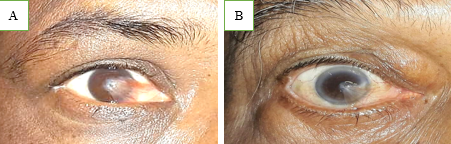
Pterygium is derived from the ancient Greek word Pterygion, which means wing. The proliferation of triangular wing-shaped fibrovascular subconjunctival tissue is non-malignant and slow-growing. It can creep the cornea (involving bowman's membrane and superficial stroma), thus disturbing the vision. It is the degeneration of conjunctival connective tissue induced by UV light. It is more commonly seen in advancing age and in people living in tropical and subtropical areas, which are exposed to dry, dusty, sunny, and windy climates and in people who are more exposed to UV light (Farmers, Arc welders etc.). It doesn’t hamper vision until it involves the visual axis. Most of the patients approach doctors for cosmetic improvement. It generally develops from a pinguecula and is usually unilateral; it mainly involves the nasal limbus ([Figure 1]) and rarely affects the temporal limbus alone ([Figure 2]). However bilateral pterygium are not uncommon ([Figure 3]).
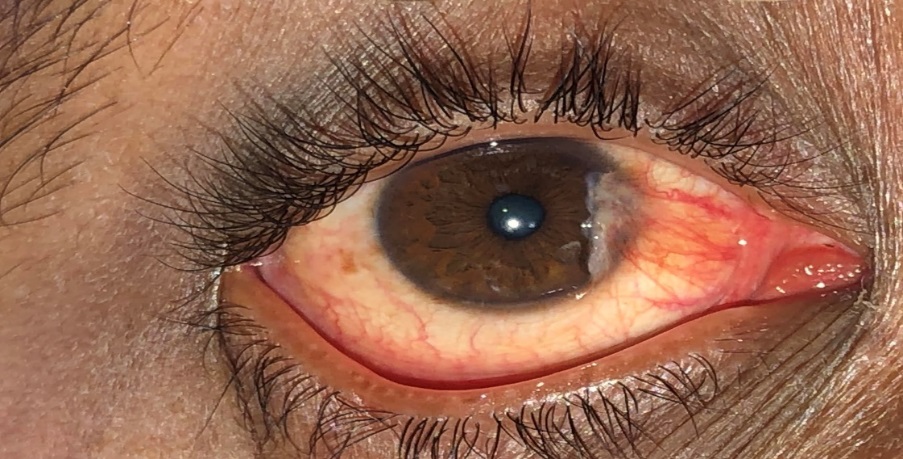
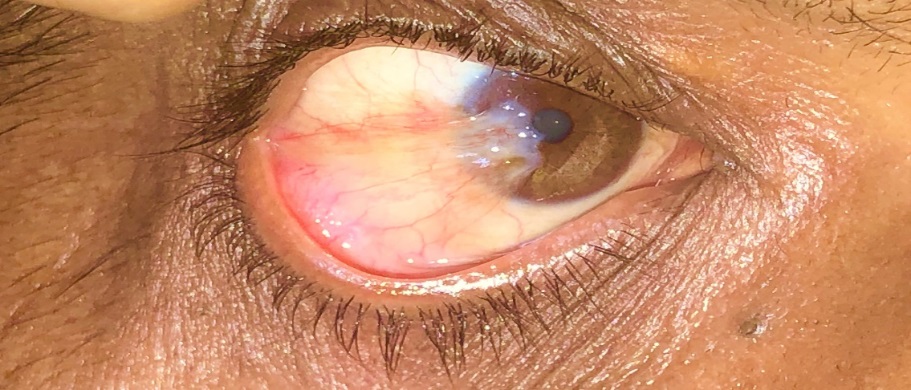
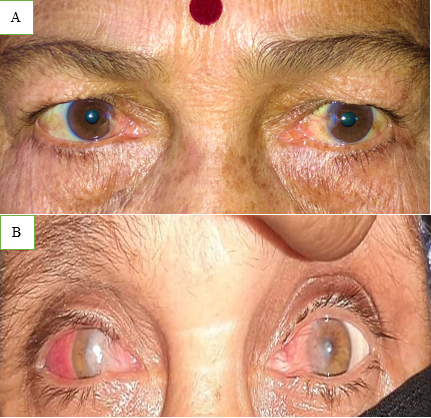
Types of Pterygia –
Progressive pterygium is thick, fleshy with pronounced vascularity, growing and can extend close to the visual axis. Cap (opaque infiltrative spot) and Stocker's line (a brownish iron line on the cornea in front of the apex) can be seen.
Atrophic (stationary) is thin and attenuated with poor vascularity. Cap is absent.
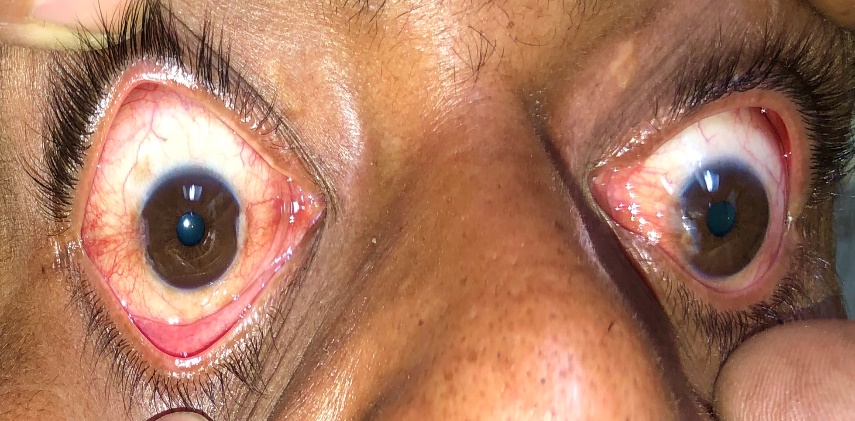
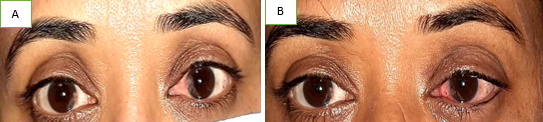
Primary double pterygium (both nasal and temporal limbus involvement) ([Figure 4], [Figure 5]) , Pseudo pterygium (adhesion of a fold of conjunctiva to the peripheral cornea, which is usually unilateral, stationary and at any meridian), Recurrent pterygium (more width, more scarring of tissue, seen after primary treatment of pterygium) and Malignant pterygium (rare, a type of recurrent pterygium with restriction of ocular movements on the opposite side) are the other types.

Pterygium is usually asymptomatic but when growth is significant, a patient may complain of discomfort, foreign body sensation, irritation, redness, inflammation, dryness, lacrimation, decreased visual acuity, and diplopia on lateral gaze, acquired irregular or with the rule of astigmatism. Significant loss of vision may occur when pterygium obscure the visual axis ([Figure 6]).
The treatment of pterygium has evolved over the years. The recurrence rate after pterygium surgery is relatively high, ranging from 2% to 40%. Following are the current treatment modalities available for the management of pterygium:- bare sclera with intraoperative mitomycin-c, bare sclera with beta irradiation, sliding or rotational conjunctival autograft, conjunctival autograft, limbal conjunctival autograft (LCAG), amniotic membrane transplant, anterior segment indocyanine green angiography with conjunctival autograft transplantation, air-assisted pterygium surgery, suture less small incision pterygium surgery with fibrin tissue glue, use of antimetabolites like thiotepa, 5-fluorouracil (5-FU), mitomycin-c (MMC), deep anterior lamellar keratoplasty, medical management with Avastin.
Aetiology and Epidemiology
Pterygium shows a worldwide distribution, but it is more common in warm and dry climates.[1] In tropical areas, prevalence is as high as 22% than in latitudes above 40 degrees, where prevalence is less than 2%.[2], [3] There are some risk factors for the development of pterygium. In a large group of studies, a strong positive correlation between ultraviolet radiation exposure (both UV-A and UV-B) and the development of pterygium has been established.[2], [3], [4], [5], [6] The role of ultraviolet radiation in the development of pterygium is further supported by the systemic associations of pterygium that includes basal cell carcinoma (BCC),[7] porphyria cutanea tarda (PCT),[8] polymorphous light eruption[9] and xeroderma pigmentosum.[10]
Tear film abnormalities leading to dryness of the cornea and conjunctiva in the interpalpebral fissure can lead to new fibroblast growth. In trade wind belts, dryness of the tear film by wind devitalizes tissues of the medial third of the palpebral aperture, allowing actinic radiation to damage the conjunctival and corneal epithelium and bowman’s membrane.[11]
It has also been suggested that repeated irritation at the limbus due to prolonged ultraviolet exposure can lead to formation of altered proteins in the bowman’s membrane, which can act as an angiogenesis factor, thus leading to vessel ingrowth and the formation of pterygium.[12]
Prevalence of pterygium is higher among men and increases with age, highest between 20 and 49 years of age.[13] Recurrences are more frequent in young adults than in older individuals.[14] Genetic predisposition to pterygium may exist in northern climates, where pterygium is exclusively seen in fishers and rural workers. These families have shown a dominant mode of inheritance, but it may be due to shared environmental factors and occupations.[15] Genetic predisposition is also supported by the increased expression of vimentin and p53 by the keratoblasts, which indicates cellular migration or a migrating limbus. These studies support that the altered limbal epithelial stem cells initiate pterygium's cellular origin.[16], [17] Pterygium predominantly has a nasal location, as the sunlight passing laterally through the cornea undergoes refraction without any obstruction and is focused on the medial limbus while the light passing medially is obscured by the nose; this reduces the intensity of light focusing on the temporal limbus. [6], [18]
Classification
The clinical appearance of the pterygium depends on its state of growth. [19] Mature pterygium is raised, triangular, with its based on the para-limbal conjunctiva and its apex towards the centre of the cornea. Anatomically, pterygium has the following parts: Fuchs patches, Stocker's line, hood, head, body, base, superior edge, and inferior edge. For evaluating the severity of pterygium, the following parameters must be observed: -
1) Length of encroachment onto the cornea is the most important indicator of severity. More is the encroachment, and more will be the visual disturbances like induced astigmatism, corneal irregularities, light scatter, or pupil obscuration. According to this parameter, pterygium can be classified into four stages: -
Stage 0- Pinguecula, posterior to the limbus.
Stage 1- pterygium is restricted to the limbus.
Stage 2- pterygium only marginally invades the cornea.
Stage 3- pterygium is between the limbus and pupillary margin.
Stage 4- pterygium is central to the pupillary margin.
2) The base width of the pterygium is usually measured with callipers as the chord length of the corneal limbus involved. Based on the size of ptrygium from the limbus pterygium may be:-
Grade 1: 0-2 mm
Grade 2: 2-4 mm
Grade 3: > 4 mm
3) The translucency of the pterygium tissue is based on the visibility of the underlying episcleral vessels. [20]
Grade I: Atrophic pterygium – episcleral vessels clearly visible.
Grade II: Intermediate pterygium – episcleral vessels partially visible.
Grade III: Fleshy or opaque pterygium – episcleral vessels not visible.
Pathogenesis of Pterygium
Pterygium is characterised by elastotic degeneration of the collagen and fibrovascular proliferation, with an overlying intact epithelium. On the H & E stain, abnormal collagen of the elastotic degeneration shows basophilia. Also it stains with elastic tissue but it is not actual elastic tissue as it is not digested by elastase . These lesions are characterised by cellular proliferation, tissue remodelling and neovascularisation. When these growths are removed, there is residual corneal scarring as pterygium leads to the destruction of the bowman’s membrane. Although the pathogenesis of pterygium is unclear but ultravoilet radiation is the initiating factor in developing pterygium.[1], [3], [6], [14], [18], [21], [22] Light and electron microscopic studies shows that the elastotic changes in the pterygium are like the actinic degenerative changes seen in chronic UV- exposed skin. [23] Some studies have demonstrated the expression of metalloproteinases( MMPs) and their inhibitors at the advancing edges of the lesions. [24], [25], [26] MMPs may play a significant role in tissue remodelling, invasion and the destruction of the bowman’s layer associated with pterygia.
Pterygium is a fibrovascular growth and epithelial hyperplasia originating at the limbus. These modified limbic cells migrate towards the cornea by an active process associated with cell growth, tissue remodelling, angiogenesis, and inflammation. UV light seems to be the initial trigger; it activates epithelial cells near the limbus to produce cytokines (IL-6 and IL-8) and growth factors which cause inflammation, fibrovascular proliferation, angiogenesis, anti-apoptosis and tissue remodelling such as destruction of bowman’s membrane, a marker of pterygium invasion. Following factors involved in pathogenesis of pterygium.
Inflammatory
Goblet cell distribution
Chronic inflammatory reactions are present in all pterygium specimens.[27] There is a marked difference in density and distribution of goblet cells in pterygia and normal conjunctiva, irrespective of age and demographic features.
T-lymphocyte subpopulations and inflammatory markers
Pterygium specimens show infiltration of T-lymphocytes (CD4 and CD8) in both the conjunctival epithelium and substantial propria. CD4/CD8 ratio is 0.33 and 1.34 for pterygium epithelium and pterygium substantia propria respectively.[28], [29] There is a higher rate of ICAM-1, VCAM-1 positive cells and HLA-DR positive cells in pterygium samples compared to normal samples.[30] A significant positive correlation is found between Metallothionein (MT) expression and lymphocyte subpopulations (T4, T8), macrophages (CD68), Langerhans’ cells (S100) and the proliferation-associated indices (PCNA, Ki67) found in pterygium and no such correlation is found in pinguecula and normal conjunctiva.[31]
Cyclooxygenase-2 expression in primary and recurrent pterygium
COX-2 is expressed in primary and recurrent pterygia specimens. There is a statistical difference between COX-2 expressions in the surface epithelium, stromal fibroblasts, vessels and inflammatory cells in recurrent pterygia tissues and control tissues.[32]
Molecular genetics
BPDE (BaP 7,8-diol 9,10-epoxide) forms a BPDE-N2-DG adduct by targeting deoxyguanosine resulting in p53 mutations. [33] p53 gene mutation and p53 protein overexpression are detected in the epithelium of primary and recurrent pterygium, [34] suggesting pterygium is a growth disorder rather than degeneration.
UV exposure
UV radiation is a major risk factor in the etiopathogenesis of pterygium as it causes oxidative DNA damage. 8-OHdG (8-hydroxyguanosine) is a sensitive and stable marker of the degree of DNA damage and p53 protein (tumour suppressor gene) is a significant cell stress regulator. UV light promotes oxidative stress and causes a mutation in the p53 gene leading to cellular proliferation. Pterygium shows limited local invasion and inability to metastasise, but despite its benign clinical course, it shows the concomitant presence of altered p53 in 8-OHdG - immunoreactive cells that leads to apparent genomic instability. [35]
Stem cells
Adult stem cells from bone marrow have been postulated in the pathogenesis of pterygium as proven by immunohistochemical staining with various stem cell markers. AC133 and CD34 positive cells are found in the basal epithelium, while c-kit cells and STRO-1 positive cells are found in the stroma. There is an abnormal production of growth factors and cytokines in response to inflammation to recover from cellular damage caused by various environmental triggers. If the response is excessive, limbus basal cells will be changed to abnormally altered pterygia cells. The excessive wound healing process and remnant altered cells are also responsible for recurrence. [36]
Hypoxia
Hypoxic conditions in the nasal limbus during the early stage of pterygium may lead to the recruitment of endothelial progenitor cells, as assessed by anterior segment fluorescein angiography (FAG). [37]
Systemic and local cytokines
Systemic and local cytokines like Substance-P (SP), vascular endothelial growth factor (VEGF), stem cell factor (SCF) is increased in pterygium groups than in normal controls. They show positive correlations with circulating CD-34 (+) and c-kit (+) mononuclear cells (MNCs), which are also elevated in pterygium groups.
Strong progenitor cell marker immunoreactivities are found in pterygium as analysed by immunohistochemistry (IHC). EPCs (endothelial progenitor cells) might be involved in developing pterygium and ocular hypoxia triggers it by recruiting EPCs from the bone marrow via the production of systemic and local cytokines. [37]
There is enormous evidence of immunologic aetiology of pterygium which provides the basis for the use of immuno-modulator drugs in the treatment of pterygium, for preventing its recurrence, and the use of conjunctival autografts and amniotic membrane in the treatment of recurrent pterygium.
Differential Diagnosis of Pterygium
Some pathologies of the peripheral cornea and limbus have a similar morphological appearance as that of pterygium; therefore, differential diagnosis is required.
Pseudo pterygium
Pseudo pterygium is a fibrovascular scar arising in the bulbar conjunctiva that extends onto the cornea. It arises from previous ocular surface inflammation of various origins such as chemical or thermal burns, autoimmune conditions, trauma, cicatrizing conjunctivitis, or peripheral corneal ulcers. It is the sectorial conjunctivalization of the corneal surface and can occur in any corneal zone. It should not be removed until it approaches the optical axis. Some differentiating features from pterygium are lack of adhesion to the limbus (so a probe or muscle hook can easily be passed underneath it, which is usually not possible with pterygia), lack of organisation into a cap, head and body and occurrence outside the interpalpebral space.
Pinguecula
Pinguecula – small, yellowish, raised nodules that appear on the bulbar conjunctiva or limbus in the intrapalpebral space. It is usually asymptomatic but can become inflamed with symptoms like itching, burning or mild pain. Surgical excision is rarely indicated, but if done, it doesn’t recur.
Limbal dermoid
Limbal dermoid, phlyctenular keratoconjunctivitis, conjunctival papilloma, conjunctival lymphoma, squamous cell carcinoma of the limbus, nodular episcleritis, Bowen’s epithelioma are other differentials but can easily be distinguished from a pterygium.
Treatment
Role of 2% lidocaine Gel in pterygium surgery
Topical anaesthesia has many advantages over injected anaesthetics like less discomfort to the patient while administering, faster recovery and less chances of complications like chemosis, diplopia, globe perforation, retrobulbar haemorrhage and optic nerve injury, which can occur in injected anaesthesia. [38]
In pterygium surgery, anaesthesia via topical administration of 2% lidocaine gel can be used as an alternative to subconjunctival injection of 2% lidocaine solution. [39] Topical anaesthesia by 2% lignocaine gel is a safe and effective way of providing anaesthesia with no pain and discomfort for the patient while administering and no observed ocular surface toxicity. [40] Lidocaine 2% gel is applied topically 5 minutes before the pterygium surgery in the inferior conjunctival fornix followed by every 10 minutes during the surgery. Lidocaine 2% gel has advantages like sustained effect of drug due to its longer contact time with the ocular surface and lubricating effect of gel, which is beneficial for the cornea. There are also some disadvantages of topical gel formulation like frequent administration of doses prior to and during the surgery, shorter anaesthetic effect, and chances of cumulative toxicity; however, no ocular surface toxicity has been reported.
A study showed that 60% of patients experience clinically significant pain after pterygium surgery. This pain is both corneal and conjunctival in origin. Topical NSAIDs and anaesthetic drops have been applied to reduce postoperative pain after ocular surface surgeries. However, topical NSAIDs have a slower onset of action, are ineffective in controlling acute pain, and show corneal toxicity. [41] Topical anaesthetic drops can reduce postoperative pain, but frequent installation is required due to its shorter contact time with the ocular surface, and prolonged application can be associated with ocular surface infections. [42] Lidocaine 2% gel application can be used to reduce postoperative pain as it is more convenient due to its sustained duration of action and fewer frequent application. [43] It was found to be superior to tetracaine eye drops in controlling immediate postoperative pain. [44]
Pterygium surgery
Surgery for pterygium is done in an outpatient setting under topical or local anaesthesia, if required under sedation. The surgery aims are to restore the standard, topographically smooth ocular surface. Multiple techniques have been developed for pterygium surgery across the years.
Bare sclera technique
Pterygium is excised and removed from the cornea to make it as smooth as possible. Tenon’s capsule is excised beneath the pterygium, leaving an area of the exposed bare sclera. Light thermal cautery is applied over the sclera for haemostasis. No sutures are used to close the conjunctiva. This technique has higher recurrence rates than other procedures, 5-68% with primary pterygium and 35-82% with recurrent pterygium.
Simple or direct closure
Free edges of the conjunctiva are sutured together with fine, absorbable sutures. This technique is only valid if the conjunctival defect is minimal. It can be used for the removal of pinguecula. Dellen (thinning of the cornea following dehydration) is the most common complication with this technique. The recurrence rate ranges from 45% to 69%.
Sliding flap technique
L-shaped incision is made to the adjacent conjunctiva to allow conjunctival flap to slide over the defect and close with interrupted and/or running sutures. Flap retractions and cyst formation are some frequent complications. Recurrence rate ranges from 0.75% to 5.6%.
Rotational flap technique
U shaped incision is made in the superior conjunctiva adjacent to the wound to make a tongue of the conjunctiva rotated into place. It is done to prevent recurrence and provide a smooth limbal surface for proper tear film distribution. The reported recurrence rate ranges from 4-6%.
Free conjunctival autograft [45], [46], [47], [48], [49], [50]
This technique manages primary advanced and recurrent pterygium.[45] The recurrence rate with this technique is only 2-5%. Pterygium is dissected and excised with all the subconjunctival tissue as possible, taking care not to damage the medial rectus tendon (in case of nasal pterygium). The head of the pterygium attached to the cornea is removed by blunt dissection using a crescent knife and polishing the cornea with no sharp side of the knife. Air-assisted dissection, [51] a new technique is used for pterygium excision in which the air is injected with a 30-gauge needle into the side of the cap of the pterygium head to make a dissection plane between the pterygium head and cornea. Incomplete air dissection leads to problem in scraping off the pterygium from the cornea. Conjunctival autograft is taken from the superotemporal bulbar conjunctiva of the same eye because it has been less exposed to UV light during the lifetime as it has been covered by the upper eyelid and thus has less histological modifications. It also has fewer chances to grow into a differentiated tissue when placed in a new location. This free graft is excised according to the size of the epithelial defect and sutured or glued into place. Handling this free graft is the most crucial step in the whole surgery and mishandling or upside-down graft placement leads to recurrence in most cases. The main advantage of this surgery is the preservation of limbal stem cells that are responsible for good corneal, limbal and conjunctival healing. This procedure is also known as “Minimally invasive pterygium surgery” or PERFECT surgery (Pterygium Extended Resection Followed by Extended Conjunctival Transplantation). Common complications of this surgery are graft failure, granuloma formation, conjunctival infection, suture detachment, sustained redness at the graft site and recurrence of pterygium.
For moderate to severe pterygia, amniotic membrane transplants can also be used.[52], [53], [54] Conjunctival autograft can be secured with interrupted, absorbable sutures, or fibrin tissue glue can also be used to seal it with the underlying sclera.
All specimens of excised pterygium should be sent for histopathological examination (HPE) to look for ocular surface squamous neoplasia (OSSN), as the rate of OSSN is in sequential pterygium specimens is reported to be as high as 9.8%. [55]
Fibrin sealant v/s conjunctival autograft
Cohen et al. first described using an organic tissue adhesive in the fixation of conjunctival autografts in 1993. [56] Shaw’s technique is used for the application of conjunctival autograft. [57] Beriplast P and Tisseel Duo Quick are the commercial products used as fibrin sealants.
Mechanism of Action of Glue – Glue can’t work on intact corneal or conjunctival epithelium. [58] Glue has two parts, one part has fibrinogen mixed with factor XIII, and aprotinin and another part has a thrombin-CaCl2 solution. Both parts are mixed in equal quantity, and by the action of thrombin, fibrinogen is converted to fibrin monomers which, by cross-linking, leads to fibrin clot. The concentration of thrombin decides coagulation time. Low thrombin concentrations (4 NIH-U/ml) have slow clotting compared to high thrombin concentrations (500 NIH-U/ml) which have rapid clotting.
Advantages of glue over suture– Shorter surgical time, lesser pain, lesser inflammation and lesser recurrences are some advantages of using glue over sutures. A randomised, prospective study found surgical time to be much lesser in the use of glue over sutures. [59] Another study found that pain was remarkably lower when the glue had been used compared to sutures, and the average surgical time was 9.7 minutes (range 6-13) for glue, and 18.5 minutes (range 12-30) for sutures. Since the surgical time is lesser, the inflammation would be lesser and recurrence depends on the amount of inflammation; thus, the recurrence would also be lesser for glue than for sutures. Recurrence is the regrowth of fibrovascular tissue 1 mm past the corneoscleral limbus. According to one study, the recurrence rate is 5.3%, the reoperation rate is 1.2%, and the recurrence rate is 13.5%; the reoperation rate is 3.3% in the glue groups, and suture group respectively. [6] At 1yr follow-up, some studies found the recurrence rate to be twice with sutures (10%) than glue60,61. One study found the recurrence rate to be 3.7% in the Tisseel group compared to 20% in the sutured group. Three months is the average recurrence time. [60]
On histology, foreign body granulation tissue is not seen with tissue glue but from day 15-45 in the Vicryl sutured group. Tissue glue is not seen under the conjunctiva after 15 days.
Complications
Subconjunctival haemorrhage, graft dehiscence, infection, discomfort, dry eye, transient transplant oedema, and persistent corneal epithelial defects are some known complications that can occur with the use of glue. [61], [62] Subjective symptoms like postoperative pain, foreign body sensation, tearing, and discomfort are much lesser in fibrin glue group than in the suture group. [61] In 50% of patients, these symptoms decrease after one week of surgery, and all these symptoms disappear within 2 weeks of surgery in all the patients. [62]
Adjunctive Therapy for Surgical Interventions
Many adjunctive therapies like mitomycin C, corticosteroids, thiotepa, interferon-alpha – 2b, beta irradiation, 5-FU are being used to decrease the risk of recurrence after surgical removal of pterygium. Judicious use of these drugs is essential as they all have side effects.
Mitomycin C
Recurrence occurs due to the proliferation of fibroblasts; therefore, mitomycin c (MMC) is used to reduce fibroblast proliferation and recurrence after pterygium surgery. [63] MMC is an antibiotic-antineoplastic agent isolated from Streptomyces caespitosus that inhibits DNA synthesis. Several positive epithelial cells for Ki-67 antigen are not related to pterygium recurrence, and MMC doesn’t reduce them but decreases fibroblast nucleus volume in the pterygium. [64] MMC can be used preoperatively, intraoperatively, and postoperatively with any techniques of pterygium surgery including the bare sclera, direct conjunctival closure, sliding, rotational conjunctival graft and conjunctival autograft. Subconjunctival injection of 0.1ml of MMC 1 month before pterygium surgery leads to reduced vascularity in the pterygium as MMC inhibits fibrovascular activity in the pterygia stroma resulting in decreased risk of pterygium recurrence. [65] Intraoperatively 0.02% MMC is applied for three minutes after pterygium excision. Sliding conjunctival graft and direct conjunctival closure with MMC have similar recurrence rates. [66] Likewise, conjunctival rotational autograft with MMC is equally effective as limbal conjunctival autografts. [67] Postoperatively, 0.02% MMC eye drops can be used twice daily for two weeks. Recurrence rates for intraoperative application of MMC are 3-25% and 5-54% for postoperative application. [68], [69] MMC reduces recurrence, but it has some complications like scleral thinning (Dellen) and necrosis, pyogenic granuloma, perforation of the eye, cataract, glaucoma, cataract, iritis, corneal oedema, irreversible damage to basal epithelial and limbal stem cells, that limits its use. [70] These side effects are dose dependent. Scleral thinning is the most common complication of MMC use. There is a significant endothelial cell loss of 6% after one month of surgery with MMC, which reduced to 4% three months after surgery. Endothelial polymegathism is seen and several hexagonal cells reduced after pterygium surgery with MMC. [71] MMC slows down corneal epithelialization and extends epithelial and stromal oedema seen postoperatively both in the centre and periphery of the cornea. In vivo confocal microscopy shows signs of punctate keratitis for two weeks in the central cornea after application of MMC, but these changes are reversible four weeks after use of 0.02% MMC. [72] MMC also causes a decrease in goblet cell density leading to decreased mucin production and dry eye. Pterygia, which is resistant to treatment and recurs acutely can be managed with topical application of MMC. [73] Bare sclera pterygium excision with excimer laser and the local application of MMC shows promising long-term results in preventing recurrence. [74] Another study shows no significant difference in pterygium recurrence rate between intraoperative and postoperative application of 0.02% MMC following surgical excision, but the complications were comparatively less for intraoperative application than for postoperative application. [75]
Thiotepa
Thiotepa (triethylenethiophosphoramide) is a nitrogen mustard alkylating agent that inhibits mitosis in all rapidly proliferating cells. It is used as an adjunctive agent to reduce the postoperative recurrence of pterygium. It is a radiomimetic agent that inhibits the proliferation of vascular endothelial cells at the operation site. [76] Its most common strength is 1:2000, given topically every three hours, starting two days postoperatively for a total duration of 6 – 8 weeks. The recurrence rate is reported between 0 – 16% when excision is followed by thiotepa therapy. [77] Complications of thiotepa include early and late onset poliosis, periorbital skin depigmentation, prolonged conjunctival injection, irritation, allergic reactions, black pigmentation of the conjunctiva and scleral perforation. [77] Periorbital skin depigmentation that can be permanent is the most common cause of the withdrawal of this drug in the postoperative management of pterygium.
5-fluorouracil
5-FU is an antiproliferative agent that decreases fibroblastic activity by inhibiting the thymidylate synthetase enzyme, which converts ribonucleotides to deoxyribonucleotides, thus preventing DNA synthesis. It acts selectively on the cell cycle's synthesis (S) phase, so cells in the synthesis phase are only inhibited, thus allowing other cells to continue to increase after exposure to 5-FU. 5-FU prevents recurrence as intralesional injection of 5-FU stops the progression of impending recurrent pterygia, resulting in an excellent surgical scar and helping to avoid repetitive surgery. Topical use of 5-FU causes epitheliopathy, ocular surface inflammation, pain, and dry eye. These complications are mild and are usually reversible with lubricants. Limited studies and variable recurrence rates limit its use. [78]
Corticosteroids
Controlled clinical trials have not confirmed the use of topical corticosteroids to prevent pterygium recurrence. Postoperative topical corticosteroids inhibit inflammation and may reduce revascularization, thereby preventing pterygium recurrence. However, long term use has side effects like cataracts, glaucoma, and ocular hypertension.
Interferon alpha
Interferons are glycoproteins that have antiproliferative and antiviral effects. A recombinant form of interferon alpha-2b (IFN-a-2b) is used in treating conjunctival intraepithelial neoplasia, conjunctival papilloma and as a lone therapy for early recurrent pterygium. However, further studies are required in this research. [79]
Beta radiation
The most common beta emission radioactive substance used in treating pterygia and other neoplasms of the eye surface is Strontium-90 (Sr-90). [80], [81] It delivers 2500 cGy to the scleral surface at a dose rate of 200-250 cGy/min. [82] Most common dosage is 15Gy in single or divided doses. Postoperative 90Sr irradiation of pterygium after surgery represents a safe and effective treatment option to prevent disease recurrence. [80] Beta irradiation reduces mitosis in rapidly dividing vascular endothelial cells, thus inhibiting the proliferation of fibroblasts and recurrence. Therapy with beta irradiation acts when it is applied in close contact with the tissue and has the advantage of minimal tissue penetration. It acts on cell cycle controller p53 and inhibits the proliferation of tenon’s fibroblasts which enter a period of growth arrest but do not die. [81] Radiotherapy is used with the bare sclera technique in which the radioactive applicator is placed in contact with the bare sclera. [80] Temporary radiotherapy-induced side effects like moderate conjunctivitis, local pain, visual disturbance, photophobia, or an increase in tear flow were observed in 15.2% of patients. [83] Due to the non-selective action of beta radiation, repair processes might get delayed. Significant long-term complications include cataracts, conjunctival telangiectasia, corneal epithelial defects, corneal and scleral thinning, and necrosis, scleritis, endophthalmitis, scleromalacia, symblepharon and corneal scarring. [81] Scleral necrosis and endophthalmitis are the most severe complications; late scleral melting is seen in around 13% of cases. Due to these harmful side effects, beta irradiation therapy should be used with caution. Recurrence rates range from 4.3-35%, with bare sclera or simple conjunctival closure technique. [84] Non-surgical, exclusive Sr/Y-90 beta irradiation up to a total dose of 50 Gy divided into four fractions one week apart can be given preoperatively for the treatment of pterygium. It reduces pterygium size in all cases, without any recurrent growth or any late side effect. [85]
Cyclosporine A (CsA)
It is a cyclic non-ribosomal peptide composed of 11 amino acids produced by the fungus. It is an immunosuppressant drug widely used in post-allogenic organ transplants to reduce the risk of organ rejection. It binds to cytosolic cyclophilin (immunophilin) of immunocompetent T lymphocytes and inhibits lymphokine production and interleukin release, leading to reduced function of effector T-cells. Topical cyclosporine A is used in many ocular surface disorders and dry eye. A study shows that topical use of 0.05% CsA for three months following pterygium excision and use of conjunctival autograft leads to less recurrence rate (3.4%) compared to the control group (17.9%). CsA group showed significantly fewer postoperative complications, e.g. graft scarring, tenon’s granuloma, postoperative pain and fibrovascular proliferation. It is proposed that postoperative application of 0.05% CsA topically following primary excision of pterygium is safe and efficient. [86]
Bevacizumab (Avastin)
It is a humanized monoclonal antibody and an angiogenesis inhibitor. It inhibits the function of vascular endothelial growth factor (VEGF), a natural protein that stimulates new blood vessel formation. It is used as an intravitreal agent in managing choroidal neovascular membrane (CNVM) in wet ARMD, proliferative diabetic retinopathy, neovascular glaucoma, retinopathy of prematurity, diabetic macular oedema, and macular oedema secondary to retinal vein occlusions. It is proposed that pterygium development depends on a changed angiogenic stimulator-to-inhibitor ratio. Bevacizumab may inhibit neovascularization and thus, may stop the progression or prevent the recurrence of pterygium. One study showed topical bevacizumab eyedrops (25mg/ml) administered 4 times daily for 3 weeks in impending recurrent pterygium. After one year follow-up, no recurrence was observed, and symptoms were resolved with no local or systemic adverse effects. The study suggested that topical bevacizumab may be effective in preventing recurrence in a patient with impending recurrent pterygium. However, long term and well-controlled studies are required to establish its use. [87]
Management of Recurrent Pterygium
Recurrence remains a significant concern after surgical excision of pterygium. Recurrence is defined as postoperative regrowth of fibrovascular tissue crossing the corneoscleral limbus. Recurrent pterygium has increased conjunctival inflammation and accelerated corneal involvement; it is often difficult to treat. HPE shows predominant fibroblastic proliferation and neovascularization in recurrent pterygium, much more prevalent than elastotic conjunctival degeneration of primary pterygium. Studies have shown that pterygium recurs easily [88] and extensive surgery for recurrent pterygium leads to more limbal stem cell deficiency and cicatricial changes in the ocular surface. That’s why many adjunctive therapies like beta-irradiation, mitomycin C and 5-FU are being used for the treatment of recurrent pterygium.
Surgery for recurrent pterygium
Conjunctival pedicle flap or rotational conjunctival flap
Preservation of normal conjunctiva for future autografting and forming a barrier of normal conjunctival tissue adjacent to the limbus to prevent recurrent pterygium growth onto the cornea are some advantages of this technique. [89]
Conjunctival autograft
Conjunctival autograft is the standard gold technique for preventing pterygium recurrence following excision (Figure-7). Conjunctival autograft has a relatively low recurrence rate as compared to other surgical procedures for recurrent pterygium.[90], [91], [92] It acts as a barrier to the invasion of conjunctival tissue. [90] After a recurrence of pterygium, surgery should be withheld for 4-6 months to decrease inflammation, vascularization, and dry eye. A new graft is usually taken from the superior bulbar conjunctiva of the contralateral eye if previous surgery was done through the conjunctival autograft technique in the same eye. As the fibrosis is very dense, aggressive dissection is required, but care should be taken not to injure the extraocular muscles and to avoid corneal perforation if going too deep during corneal dissection. Retrobulbar or peribulbar block can be used to reduce pain during fibrosis removal. Care should be taken while moving the graft from one eye to another, not to turn it upside down. [45], [49], [50] For advanced recurrent pterygia with greater conjunctival involvement or multiple heads, this procedure is limited due to the unavailability of the remaining normal conjunctiva in the same or another eye.
Limbal autograft
According to some studies, UV light induced destruction of limbal stem cells results in pterygium. Hence, it is suggested to use limbal conjunctival autograft transplantation to prevent recurrence in either primary or recurrent pterygium. [93], [94], [95] Since recurrent pterygium is more extensive and usually requires a large conjunctival barrier to prevent another recurrence after the removal of subconjunctival fibrovascular tissue, limbal autografts are more beneficial than conjunctival autografts as they provide much larger tissue, even in frequently recurring pterygium.
Limbal allograft
Limbal allografts are obtained from the cornea of the eye bank before surgery. The period from donor’s death to transplantation surgery should be less than seven days. It reduces the height difference between the graft and the surrounding cornea and sclera and aids epithelial supply to the denuded area from the limbal allograft. The epithelial side of the graft is used for surgery. It is trimmed to cover the limbal deficient area and sutured to the sclera. [96] Amniotic membrane transplantation is used with limbal allograft to ensure epithelialization of the excised area. Rejection of graft is the primary concern in limbal allograft transplantation.
Amniotic membrane transplantation
Amniotic membrane transplantation helps epithelialization and reduces inflammation, vascularization, and scarring. Recurrence rates for amniotic membrane transplantation after pterygium excision are 10.9% and 37.5% for primary and recurrent pterygia, respectively.[92] Advantages of amniotic membrane transplantation in recurrent pterygia are its ability to restore large excised areas, which is impossible with conjunctival autografts, in conditions of scarred conjunctiva from previous surgery or where the conjunctiva is being kept for possible glaucoma-filtering surgery.[54] It is helpful for extensive conjunctival defects as in double-headed pterygium and for treating multicurrent pterygia with symblepharon and motility restriction. [97]
Buccal mucous embrane grafts and skin grafts
Split thickness buccal mucosal graft with beta-irradiation is used in complex recurrent pterygium cases in which there is insufficient autologous conjunctiva and conjunctival shortening with restricted eye movements. [98] It decreases recurrence rates in secondary recurrent pterygia and postoperatively presents with an acceptable “white” eye, [99] but the cosmetic results of skin grafting are not very good. Due to limited number of studies, it is not being accepted much.
Lamellar and penetrating keratoplasty
If significant corneal thinning is present, lamellar keratoplasty is indicated for restoring the normal ocular surface integrity. It acts as a barrier to the regrowth of pterygium. [100], [101], [102] The use of lyophilised donor tissue for lamellar keratoplasty in recurrent pterygia is booming, with only one recurrence in 13 eyes. [102] A penetrating keratoplasty is indicated in severe cases where the visual axis is involved due to thinning and scarring to rehabilitate the eye visually. [102]
Adjunctive Therapy
Combined excisional techniques with various adjunctive treatment modalities have been suggested for both advanced primary and secondary recurrent pterygium as they lower the recurrence rates following excision. Beta-irradiation, mitomycin C (MMC), thiotepa, 5-FU and laser therapy have been employed as adjunctive therapy for secondary recurrent pterygium.
Laser therapy
Laser therapy works on the principle of therapeutic thermolabilities effect. It is the conversion of laser light into heat energy. Argon laser is used for laser therapy and emits coherent blue-green light of 488-515nm wavelength. It prevents neovascularization tufts in recurring pterygia from growing onto healing cornea and pterygium bed, thus preventing recurrence of pterygium. It is applied under a slit lamp, and thus, it provides a selective and well-controlled ablation to the pterygium bed without harming surrounding tissues. It can’t be used in bedridden and head tremors patients and can be used with difficulty in nervous, less motivated, and young cases. The recurrence rate of laser therapy is unknown. [103]
Current Treatment Modalities
Lasers
Excimer photokeratectomy [104]
Phototherapeutic keratectomy with an ArF: Excimer laser after pterygium excision using the bare sclera technique and mitomycin C 0.02% eyedrops is a safe method with good functional results and a low recurrence rate.
Photodynamic therapy
Verteporfrin [105]
It leads to successful photothrombosis of pterygium vascularization immediately after treatment with a 689nm laser delivered onto the pterygium after verteporfin infusion. In all cases, regression is shown by a scarring reaction of the corneal apex with complete or partial disappearance of vascularity with no appropriate side effect. It is indicated for patients with low-medium grade pterygium who refuse surgery. Multiple sessions may be required.
Carboxyfluorescein ester BCECF-AM [106]
PDT with carboxyfluorescein ester BCECF-AM as an adjunctive treatment modality to reduce pterygium recurrence is being challenged.
Irradiation with 32P source [107]
Applicator for irradiation with betas of a 32P source has been successful in treating pterygium. It has a lower maximum energy (1.71 MeV) than 90Sr/Y (2.27MeV) but has average energy like that of 90Sr/Y. [107] Surface dose distribution (up to 0.75 mm depth) is very similar to 90Sr/Y, and beyond 0.75 mm depth, the 32P doses decrease with depths more rapidly than 90Sr/Y doses, thus sparing the lens better than the 90Sr/Y applicator.
Recognition of remnant by Concanavalin A (ConA) [108]
Concanavalin A (ConA) lectin binds to the pterygial surface and is used to detect the recurrence or remnants of pterygium after surgical excision. It is used to detect remnants of pterygium in postoperative patients and recurrences in early pre-clinical stages through the visualization of fluorescent ConA bound to the pterygial surface.
Radiofrequency unit [109]
It is used for harvesting conjunctival autografts in pterygium surgery and it lessens fibroblast proliferation and thus gives a very good surgical scar.
Medicines in the Pipeline
Curcumin [110]
Effect of curcumin on proliferation and apoptosis of human pterygium fibroblasts (HPF) in culture was researched to find a new way to prevent the recurrence after pterygium surgery. It was proposed that curcumin could remarkably inhibit the proliferation of HPF, make HPF arrest in G0/G1 phase and induce the apoptosis of HPF in a dose-and time-dependent manner. Administration of 20-80 micromol/L curcumin for 24-72 hrs significantly inhibits HPF proliferation in dose-and time-dependent manner.
Tacrolimus [111]
Fibroblast proliferation activity of regular tenon’s capsule and recurrent pterygia exposed to tacrolimus (FK506) in vitro has been tested, and the fibroblasts from pterygia exposed in vitro to tacrolimus shows a significantly lower proliferation rate than controls after one day of exposure.
Nitric oxide and superoxide dismutase [112]
Low levels of NO and SOD have been found in pterygial tissue, and these low levels may have some role in pterygium development.
Recombinant growth factor[113]
Topical EGF (epithelial growth factor) eyedrops can be used if the persistent epithelial defect is present after surgery. EGF eye drops can accelerate the proliferation and recovery of wound corneal epithelial defects, thus the wound corneal healing time is reduced to five days compared to seven days in controls.
Conflict of Interest
The authors declare that they have no conflict of interest.
Source of Funding
None.
References
- ME Cameron. Pterygium throughout the world. 1965. [Google Scholar]
- FD Mackenzie, LW Hirst, D Battistutta, A Green. Risk analysis in the development of pterygia. Ophthalmology 1992. [Google Scholar] [Crossref]
- DJ Moran, FC Hollows. Holows FC Pterygium and ultraviolet radiation: a positive correlation. Br ophthalmol 1984. [Google Scholar] [Crossref]
- I Karai, S Horiguchi. Pterygium in welders. Br J Ophthalmol 1984. [Google Scholar] [Crossref]
- HR Taylor, SK West, FS Rosenthal, B Mufioz, HS Newland. Emmen EA Corneal changes associated with chronic UV irradiation. Arch Ophthalmol 1989. [Google Scholar] [Crossref]
- MT Coroneo. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol 1993. [Google Scholar] [Crossref]
- N Kerkenezov. A pterygium survey of the far north coast of New South Wales. Trans Ophthalmol Soc Aust 1956. [Google Scholar]
- H Hammer, I Korom. Photodamage of the conjunctiva in patients with porphyria cutanea tarda. Br J Ophthalmol 1992. [Google Scholar] [Crossref]
- DC Fletcher, KG Romanchuk, PR Lane. Conjunctivitis and pterygium associated with the American Indian type of polymorphous light eruption. Can J Ophthalmol 1988. [Google Scholar]
- H El-Hefnawi, A Mortada. Ocular manifestations of xeroderma pigmentosum. Br J Dermatol 1965. [Google Scholar] [Crossref]
- R Elliott. The aetiology of pterygium. Trans Ophthalmol Soc N Z 1961. [Google Scholar]
- . A hypothesis on the pathogenesis of pterygiums. Ann Ophthalmol 1978. [Google Scholar]
- JH Hilgers. Pterygium: its incidence, heredity and etiology. Am J Ophthalmol 1960. [Google Scholar] [Crossref]
- HR Taylor, S West, B Munioz, FS Rosenthal, SB Bressler, NM Bressler. The long- term effects of visible light on the eye. Arch Ophthalmol 1992. [Google Scholar] [Crossref]
- H Forsius, A Eriksson. pterygium and its relation to arcus senilis, pinguecula and other similar conditions. Acta Ophthalmol (Copenh) 1962. [Google Scholar] [Crossref]
- A Gulani, YK Dastur. Simultaneous pterygium and cataract surgery. J Postgrad Med 1995. [Google Scholar]
- N Dushku, Reid, T W. Immunohistochemical evidence that human pterygia originate from an invasion of vimentin-expressing altered limbal epithelial basal cells. Curr Eye Res 1994. [Google Scholar] [Crossref]
- AJ Maloof, A Ho, MT Coroneo. Influence of corneal shape on limbal light focusing. Invest Ophthalmol Vis Sci 1994. [Google Scholar]
- T W Reid, N Dushku. Pterygia and limbal epithelial cells: Relationship and molecular mechanisms. Progr Retinal Eye Res 1996. [Google Scholar] [Crossref]
- DTH Tan, SP Chee, KBG Dear, ASM Lim. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjuctival autografting with bare sclera excision. Arch Ophthalmol 1997. [Google Scholar] [Crossref]
- S M Saw, K Banerjee, D Tan. Risk factors for the development of pterygium in Singapore: A hospital-based case-control study. Acta Ophthal Scand 2000. [Google Scholar] [Crossref]
- M T Coroneo, Di Girolamo, N Wakefield, D. The pathogenesis of pterygia. Curr Opin Ophthalmol 1999. [Google Scholar] [Crossref]
- ME Camereon. Histology of pterygium: an electron microscopic study. Br J Ophthalmol 1983. [Google Scholar] [Crossref]
- N Di Girolamo, P McCluskey, A Lloyd, MT Coroneo, D Wakefield. Expression of MMPs and TIMPS in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci 2000. [Google Scholar]
- Di Girolamo, N Coroneo, Mt, D Wakefield. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci 2001. [Google Scholar]
- N Di Girolamo, D Wakefield, MT Corneo, . Differential 244 expression of matrix metalloproteinases and their tissue inhibitors at the advancing pterygium head. Invest Ophthalmol Vis Sci 2000. [Google Scholar]
- D Gaton, L Reznick, M Cunitzezki, D Weinberger, I Avisar, R Avisar. Goblet cell distribution and epithelial cell morphology in pterygium. Harefuah 2006. [Google Scholar]
- RM Awdeh, JJ Destafeno, DM Blackmon, TJ Cummings, T Kim. The presence of T-lymphocyte subpopulations (CD4 and CD8) in pterygia: evaluation of the inflammatory response. Adv Ther 2008. [Google Scholar]
- U Beden, M Irkec, D Orhan, M Orhan. The roles of 29 T-lymphocyte subpopulations (CD4 and CD8), intercellular adhesion molecule-1 (ICAM-1), HLA-DR receptor, and mast cells in etiopathogenesis of pterygium. Ocul Immunol Inflamm 2003. [Google Scholar]
- MT Perra, C Maxia, A Corbu, L Minerba, R Demurtas Pjo Colombari, D Murtas. Oxidative stress in pterygium: relationship between p53 and 8-hydroxydeoxyguanosine. Mol Vis 2006. [Google Scholar]
- S Tsironi, E Ioachim, M Machera, M Aspiotis, N Agnanti, K Psilas. Presence and possible significance of immunohistochemically demonstrable metallothionein expression in pterygium versus pinguecula and normal conjunctiva. Eye (Lond) 2001. [Google Scholar] [Crossref]
- N Karahan, S Baspinar, M Ciris, CL Baydar. Kapucuoglu N Cyclooxygenase-2 expression in primary and recurrent pterygium. Indian J Ophthalmol 2008. [Google Scholar]
- TJ Lai, YY Tsai, YW Cheng, CC Chiang, H Lee, MC Chou. An association between BPDE-like DNA adduct levels and P53 gene mutation in pterygium. Mol Vis 2006. [Google Scholar]
- SD Atkinson, JE Moore, S Shah, A Sharma, RM Best, A Leccisotti. P63 expression in conjunctival proliferative diseases: pterygium and laryngo-onycho-cutaneous (LOC) syndrome. Curr Eye Res 2008. [Google Scholar] [Crossref]
- L Liu, D Yang. Immunological studies on the pathogenesis of pterygium. Chin Med Sci J 1993. [Google Scholar]
- YS Song, YH Ryu, SR Choi, JC Kim. The involvement of adult stem cells originated from bone marrow in the pathogenesis of pterygia. Yonsei Med J 2005. [Google Scholar]
- JK Lee, YS Song, HS Ha, JH Park, MK Kim, AJ Park. Endothelial progenitor cells in pterygium pathogenesis. Eye 2007. [Google Scholar]
- JS Duker, JB Belmont, WE Benson, HLJR Brooks, GC Brown, JL Federman. Inadvertment globe perforation during retrobulbar and peribulbar anaesthesia. Patient characteristics, surgical management and visual outcome. Ophthalmology 1991. [Google Scholar] [Crossref]
- H Oksuz, C Tamer. Efficacy of lidocaine 2% gel in pterygium surgery. Acta Ophthalmol Scand 2005. [Google Scholar] [Crossref]
- IS Barequet, ES Soriano, WR Green, TP O'Brien. Provision of anesthesia with single application of lidocaine 2% gel. J Cataract Refract Surg 1999. [Google Scholar] [Crossref]
- AJ Flach. Topically applied nonsteroidal anti-inflammatory drugs and corneal problems: an interim review and comment. Ophthalmology 2000. [Google Scholar] [Crossref]
- WG Marr, R Wood, L Senterfit, S Sigelman. Effect of topical anaesthetics on regeneration of corneal epithelium. Am J Ophthalmol 1957. [Google Scholar]
- H Oksuz, C Tamer. Pain Relief after Pterygium Surgery with Viscous Lidocaine. Ophthalmologica 2006. [Google Scholar] [Crossref]
- AL Young, GY Leung, LL Cheng, TT Lau, PT Lam, DS Lam. Randomised controlled trial on the effectiveness of lidocaine gel vs tetracaine drops as the sole topical anaesthetic agent for primary pterygium surgery. Eye 2008. [Google Scholar] [Crossref]
- KR Kenyon, MD Wagoner, ME Hettinger. Conjunctiva autograft transplantation for advanced and recurrent pterygium. Ophthalmology 1985. [Google Scholar]
- S Kamel. The Pterygium: its etiology and treatment. Am J Ophthalmol 1954. [Google Scholar] [Crossref]
- S M Saw, D Tan. Pterygium: prevalence, demography and risk factors. Ophthalmic Epidemiol 1999. [Google Scholar]
- S Lewallen. A randomized trial of conjunctival autografting for pterygium in the tropics. Ophthalmology 1989. [Google Scholar]
- T Starck, K R Kenyon, F Serrano. Conjunctival autograft forprimary and recurrent pterygia: surgical technique and problem management. Cornea 1991. [Google Scholar]
- SE Ti, SP Chee, KB Dear, DT Tan. Analysis of variation in success rates in conjunctival autografting for primary and recurrent pterygium. Br J Ophthalmol 2000. [Google Scholar]
- G Gulkilik, S Kocabora, M Taskapili. Ozsutcu MA new technique for pterygium excision: air-assisted dissection. Ophthalmologica 2006. [Google Scholar]
- AK Jain, R Bansal, J Sukhija. Human amniotic membrane transplantation with fibrin glue in management of primary pterygia: a new tuck-in technique. Cornea 2008. [Google Scholar]
- A Kheirkhah, V Casas, H Sheha, VK Raju, SC Tseng. Role of conjunctival inflammation in surgical outcome after amniotic membrane transplantation with or without fibrin glue for pterygium. Cornea 2008. [Google Scholar]
- H Oguz. Amniotic membrane grafting versus conjunctival autografting in pterygium surgery. Clin Experiment 2005. [Google Scholar]
- L W Hirst, R A Axelsen, I Schwab. Pterygium and associated ocular surface squamous neoplasia. Arch Ophthalmol 2009. [Google Scholar]
- R A Cohen, M B Mcdonald. Fixation of conjunctival autografts with an organic tissue adhesive. Arch Ophthalmol 1993. [Google Scholar]
- EL Shaw. A modified technique for conjunctival transplant. CLAO J 1992. [Google Scholar]
- H Red, G Schlag, G Schlag, H Redl. Fibrin sealant and its modes of application.. Fibrin sealant in operative medicine, Ophthalmology, neurosurgery 1986. [Google Scholar]
- HS Uy, JM Reyes, JD Flores, R Lim-Bon- Siong. Comparison of fibrin glue and sutures for attaching conjunctival autografts after pterygium excision. Ophthalmology 2005. [Google Scholar]
- M Farid, JR Pirnazar. Pterygium recurrence after excision with conjunctival autograft: a comparison of fibrin tissue adhesive to absorbable sutures. Cornea 2009. [Google Scholar]
- A Karalezli, C Kucukerdonmez, Y A Akova, R Altan-Yaycioglu, M Borazan. Fibrin glue versus sutures for conjunctival autografting in pterygium surgery: a prospective comparative study. Br J Ophthalmol 2008. [Google Scholar]
- HH Kim, HJ Mun, YJ Park, KW Lee, JP Shin. Conjunctivolimbal autograft using a fibrin adhesive in pterygium surgery. Korean J Ophthalmol 2008. [Google Scholar]
- L Mastropasqua, P Carpineto, M Ciancaglini. Effectiveness of intraoperative mitomycin C in the treatment of recurrent pterygium. Ophthalmologica 1994. [Google Scholar] [Crossref]
- GC De Almeida Junior, FB Frederico, KP Watanabe, TV Garcia, AY Iquejiri, PM Cury. Evaluation of epithelial cell proliferating activity and fibroblast - nuclearkariometry in recurrent pterygium treated with mitomycin C. Arq Bras Oftalmol 2008. [Google Scholar]
- YS Chang, WC Chen, SH Tseng, CI Sze, CL Wu. Subconjunctival mitomycin C before pterygium excision:an ultrastructural study. Cornea 2008. [Google Scholar]
- F De La Hoz, JA Montero, JL Alió, J Javaloy, JM Ruiz-Moreno, E Sala. Efficacy of mitomycin C associated with direct conjunctival closure and sliding conjunctival graft for pterygium surgery. Br J Ophthalmol 2007. [Google Scholar] [Crossref]
- AL Young, PM Tam, GY Leung, LL Cheng, PT Lam, DS Lam. Prospective study on the safety and efficacy of combined conjunctival rotational autograft with intraoperative 0.02%mitomycin C in primary pterygium excision. Cornea 2009. [Google Scholar]
- J Frucht-Pery, M Ilsar, I Hemo. Single dosage of mitomycinC for prevention of recurrent pterygium:preliminary report. Cornea 1994. [Google Scholar] [Crossref]
- S Hayasaka, S Noda, Y Yamamoto, T Setogawa. Postoperative instillation of low-dose mitomycin C in thetreatment of primary pterygium. Am J Ophthalmol 1988. [Google Scholar] [Crossref]
- JA Cardillo, MR Alves, LE Ambrosio. S intraoperative application versus postoperative mitomycin C eye drops inpterygium surgery. Ophthalmology 1995. [Google Scholar] [Crossref]
- I Bahar, I Kaiserman, A P Lange, A Slomovic, E Levinger, W Sansanayudh. Slomovic AR The Effect of Mitomycin C on Corneal Endothelium in Pterygium Surgery. Am J Ophthalmol 2008. [Google Scholar]
- A Zhivov, R Beck, R F Guthoff. Corneal and conjunctival findings after mitomycinC application in pterygium surgery: an in-vivo confocal microscopy study. Acta Ophthalmol 2008. [Google Scholar] [Crossref]
- RS Adyanthaya, FA Folgar, EK Akpek. Medical management of acutely recurring pterygium with topical mitomycin-C. J Cataract Refract Surg 2009. [Google Scholar]
- R Sinha, NSharma, RB Vajpayee. Long-term results after bare sclera pterygium resection with excimer smoothing and local application of mitomycin C. Cornea 2006. [Google Scholar] [Crossref]
- A Rahman, K Yahya, K S Ui Hasan. Recurrence rate of pterygium following surgical excision with intraoperative versus postoperative mitomycin-C. J Coll Physicians Surg Pak 2008. [Google Scholar]
- K Olander, H K Haik, G M Haik. Management o pterygia: should thiotepa be used?. Ann Ophthalmol 1978. [Google Scholar]
- W Kleis, G Pico. Thiotepa therapy to prevent postoperative pterygium occurrence and neovascularization. Am J 1973. [Google Scholar] [Crossref]
- P Prabhasawat, N Tesavibul, K Leelapatranura, T Phonjan. Efficacy of subconjunctival5 fluorouracil and triamcinolone injection in impending recurrent pterygium. Ophthalmology 2006. [Google Scholar] [Crossref]
- S Esquenazi. Treatment of early pterygium recurrence with topical administration of interferon alpha-2b. Can J Ophthalmol 2005. [Google Scholar]
- SB Paryani, WP Scott, JW Wells, DW Johnson, RJ Chobe, A Kuruvilla. Management of pterygium with surgery and radiation therapy. The North Florida Pterygium Study Group. Int J Radiat Oncol Biol Phys 1994. [Google Scholar] [Crossref]
- Y Nishimura, A Nakai, T Yoshimasu. Long-term results of fractionated strontium-90 radiation therapy for. Pterygia J Radiat Oncol Biol Phys 2000. [Google Scholar] [Crossref]
- IM Jurgenliemk-Schulz, LJ Hartman, JM Roesink, RJ Tersteeg, I Van Der Tweel, HB Kal. Prevention of pterygium recurrence by postoperative single-dose beta-irradiation:a prospective randomized clinical double-blind trial. Int J Radiat Oncol Biol Phys 2004. [Google Scholar]
- MP Mourtis, HK Wyrdeman, IM Jurgenliemk-Schulz, E Bidlot. Favorable long- term results of primary pterygium removal by bare sclera extirpation followed by a single 90Strontium application. Eur J Ophthalmol 2008. [Google Scholar]
- J E Mcdonald, F M Wilson. Ocular therapy with beta particles. Trans Am Acad Ophthalmol Otolaryngol 1959. [Google Scholar]
- B Pajic, RH Greiner. Long term results of non-surgical,exclusive strontium-/yttrium-90 beta-irradiation of pterygia. Radiother Oncol 2005. [Google Scholar]
- OY Tok, AB Nurozler, G Ergun, FA Kocaoglu, S Duman. Topical Cyclosporine A in the Prevention of Pterygium Recurrence. Ophthalmologica 2008. [Google Scholar] [Crossref]
- PC Wu, HK Kuo, MH Tai, SJ Shin. Topical bevacizumab eyedrops for limbal- conjunctival neovascularizationin impending recurrent pterygium. Cornea 2009. [Google Scholar]
- LW Hirst, A Sebban, D Chant. Pterygium recurrence time. Ophthalmology 1994. [Google Scholar] [Crossref]
- H Zauberman. Pterygium surgery and its recurrence. AmJ Ophthalmol 1967. [Google Scholar] [Crossref]
- DT Tan, SP Chee, KB Dear, AS Lim. Effect of pterygium morphology on pterygium recurrence in a controlled trail comparing conjunctival autografting with bare sclerae excision. Arch Ophthalmol 1997. [Google Scholar] [Crossref]
- FM Mutlu, G Sobaci, T Tatar, E Yildirim. A comparative study of recurrent pterygium surgery: limbal conjunctival autograft transplantation versus mitomycin C with conjunctival flap. Ophthalmology 1999. [Google Scholar] [Crossref]
- P Prabhasawat, K Barton, G Burkett, SCG Tseng. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology 1997. [Google Scholar] [Crossref]
- KR Kenyon, SC Tseng. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989. [Google Scholar] [Crossref]
- SCG Tseng. Concept and application of limbal stem cells. Eye 1989. [Google Scholar] [Crossref]
- V Puangsricharern, SCG Tseng. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology 1995. [Google Scholar] [Crossref]
- T Miyal, R Hara, R Nejima, K Miyata, T Yonemura, S Amano. Limbal allograft, amniotic membrane transplantation, and intraoperative mitomycin C for recurrent pterygium. Ophthalmology 2005. [Google Scholar] [Crossref]
- YF Yao, WY Qiu, YM Zhang, SC Tseng. Mitomycin C, amniotic membrane transplantation and limbal conjunctival autograft for treating multirecurrent pterygia with symblepharon and motility restriction. Graefes Arch Clin Exp Ophthalmol 2006. [Google Scholar] [Crossref]
- J Forbes, R Collin, J Dart. Split thickness buccal mucous membrane grafts and β irradiation in the treatment of recurrent pterygium. Br J Ophthalmol 1998. [Google Scholar] [Crossref]
- WW Wong. Behavior of skin grafts in treatment of recurrent pterygium. Ann Ophthalmol 1977. [Google Scholar]
- PA Laughrea, JJ Arentsen. Lamellar keratoplasty in the management of recurrent pterygium. Ophthalmic Surg 1986. [Google Scholar]
- RH Pourier, JR Fish. Lamellar keratoplasty for recurrent pterygium. Ophthalmic Surg 1976. [Google Scholar]
- M Busin, BL Halliday, RC Arffa, MB McDonald, HE Kaufman. Preserved lyophilized tissue for lamellar keratoplasty in recurrent pterygium. Am J Ophthalmol 1986. [Google Scholar] [Crossref]
- D R Caldwell. Laser surgery for primary and recurrent pterygia. Highlights of Ophthalmology. 30th anniversary Edn.. 1985. [Google Scholar]
- T Walkow, J Daniel, CH Meyer, EB Rodrigues, S Mennel. Long-term results after bare sclera pterygium resection with excimer smoothing and local application of mitomycin C. Cornea 2005. [Google Scholar]
- M Fossarello, E Peiretti, Zucca 1, MT Perra, A Serra. Photodynamic therapy of pterygium with verteporfin: a preliminary report. Cornea 2004. [Google Scholar] [Crossref]
- A Hueber, S Grisanti, Diestelhorst. Photodynamic therapy for wound- healing modulation in pterygium surgery. A clinical pilot study. Graefes Arch Clin Exp Ophthalmol 2005. [Google Scholar] [Crossref]
- YK Park, SJ Ye, IH Kim, WR Wee, MK Kim, HS Han. Potential use of P-32 ophthalmic applicator: Monte Carlo simulations for design and dosimetry. Med Phys 2008. [Google Scholar]
- JA Díaz-González, MA Mayoral-Chávez, PL Bohórquez, Torre De La, P Mdel, P Hernández-Cruz. Role of concanavalin A lectin in recognition of pterygium remnant after surgical excision: preliminary results of a prospective study. Indian J Ophthalmol 2007. [Google Scholar] [Crossref]
- JG Camara, B Dela Cruz-Rosas, LT Nguyen. The use of a radiofrequency unit for harvesting conjunctival autografts -in pterygium surgery. Am J Ophthalmol 2004. [Google Scholar]
- M Zhang, F Bian, C Wen, Hao. Inhibitory effect of curcumin on proliferation of human pterygium fibroblasts. J Huazhong Univ Sci Technolog Med Sci 2007. [Google Scholar]
- CS Carvalho, MM Viveiros, SA Schellini, JM Candeias, CR Padovani. Fibroblasts from recurrent pterygium and normal Tenon's capsule exposed to tacrolimus (FK- 506) . Arg Bras Oftalmol 2007. [Google Scholar]
- G Ozdemir, F Inanc, M Kilinc. Investigation of nitric oxide in pterygium. Can J Ophthalmol 2005. [Google Scholar] [Crossref]
- H Hong, W Zhang, P Liu, P Zhu. Effect of recombinant epidermal growth factor on corneal epithelial cells after excision of pterygium. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2001. [Google Scholar]
- Introduction
- Aetiology and Epidemiology
- Classification
- Pathogenesis of Pterygium
- Inflammatory
- Goblet cell distribution
- T-lymphocyte subpopulations and inflammatory markers
- Cyclooxygenase-2 expression in primary and recurrent pterygium
- Molecular genetics
- UV exposure
- Stem cells
- Hypoxia
- Systemic and local cytokines
- Differential Diagnosis of Pterygium
- Treatment
- Role of 2% lidocaine Gel in pterygium surgery
- Pterygium surgery
- Bare sclera technique
- Simple or direct closure
- Sliding flap technique
- Rotational flap technique
- Free conjunctival autograft [45], [46], [47], [48], [49], [50]
- Complications
- Adjunctive Therapy for Surgical Interventions
- Mitomycin C
- Thiotepa
- 5-fluorouracil
- Corticosteroids
- Interferon alpha
- Beta radiation
- Cyclosporine A (CsA)
- Bevacizumab (Avastin)
- Management of Recurrent Pterygium
- Adjunctive Therapy
- Current Treatment Modalities
- Lasers
- Photodynamic therapy
- Irradiation with 32P source [107]
- Recognition of remnant by Concanavalin A (ConA) [108]
- Radiofrequency unit [109]
- Medicines in the Pipeline
- Curcumin [110]
- Tacrolimus [111]
- Nitric oxide and superoxide dismutase [112]
- Recombinant growth factor[113]
- Conflict of Interest
- Source of Funding
