Introduction
Ocular surface squamous neoplasia (OSSN) encompasses a spectrum of epithelial squamous malignancies, that range from mild to severe dysplasia to invasive squamous cell carcinoma.1 The incidence of OSSN across the world has been reported to be 0.02-3.5 cases per 100,000, 2 with a greater frequency in regions near the equator. 3 OSSN generally presents in the late sixth to seventh decades of life, but has been seen to occur earlier in people who are immunocompromised. 4 Several risk factors have been implicated in causing OSSN which include UV light exposure, an immunocompromised state as in HIV and Human Papilloma Virus infection, 5 smoking, 6 advanced age and male gender. 7
The mainstay of treatment of OSSN involves surgical excision. However, a high rate of recurrence has been observed following surgical excision alone. Therefore, several adjunctive therapies have been described, which have been found to be effective in preventing tumor recurrence. Herein, we undertook a study of 25 eyes in 25 patients with localized primary OSSN who were treated with surgical excision and topical Mitomycin -C(MMC) as an adjunct and have reported the recurrence rate after a follow -up period of 24 months.
Materials and Methods
After seeking ethical committee approval and written consent from the patients, the study was conducted in our institute. The study conducted was a hospital based, prospective interventional study that involved 25 eyes of 25 patients with localized OSSN who presented between January 2017 to January 2020.
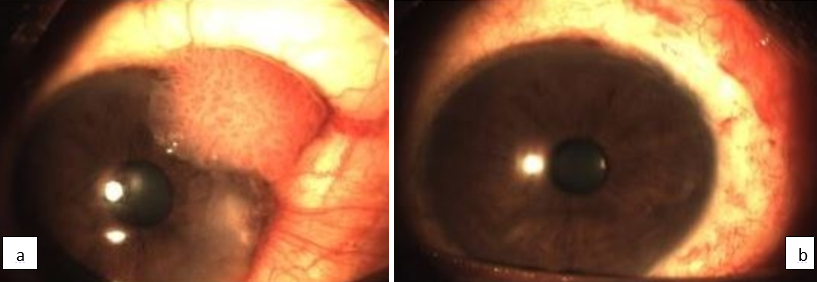
A detailed history including demographic details, duration of symptoms and risk factor exposure were recorded. A thorough clinical examination was conducted that included visual acuity assessment, a detailed anterior segment evaluation including the shape, size, mobility and extent of the lesion, any anterior chamber reaction, fluorescein staining under slit lamp biomicroscopy, fundus evaluation and lymphadenopathy. Inclusion criteria involved localized non-invasive OSSN (with associated corneal involvement as visualized on slit lamp evaluation and confirmed on histopathological examination. (Figure 1a). Patients with advanced disease were excluded from the study.
The protocol for management involved complete surgical excision of the lesion with a 2mm healthy rim and the excised tissue was sent for hisptopathological examination. The surgical excision was carried out by the same operating surgeon in all the cases. The ocular surface was left to heal or amniotic membrane transplantation done in case the defect created was more than 4mm in size. After the corneal epithelial surface healed completely, 0.04% solution of Mitomycin-C (MMC) was prepared by reconstituting a 2mg/ml MMC vial with 5ml of sterile distilled water. The solution was refrigerated after reconstitution. All patients received freshly prepared topical 0.04% MMC and were instructed to instill it four times a day as eye drops and continue for 3 cycles with 2 weeks on and 2 weeks off for 12weeks. The patients were followed up at 1 week, 2 weeks, monthly for 6 months and thereafter every 3 months for the next 24 months. At each visit a thorough slit lamp evaluation was performed along with fluorescein staining and routine examination for any corneal keratitis or erosions and to look for any tumor recurrence (Figure 1 b). The effectiveness of MMC was assessed in the form of tumor recurrence. (Table 1)
Table 1
Results
Of the 25 patients, there were 7 females and 18 males, with 25 primary OSSN as depicted in Table 1, clearly indicating a male preponderance. The mean age was 64 (SD 13) with a range of 47-87 years. Of the 25 patients who underwent surgical excision, 20 patients received 3 cycles of freshly prepared 0.04% MMC with 2 weeks on and 2 weeks off for 12 weeks. 5 patients (20%) developed an allergic response to MMC in the form of mild redness and itching, because of which they received only 2 courses of 0.04% MMC. The symptoms of MMC allergy resolved completely after discontinuation of therapy. All patients were followed up thereafter at 1 week, 2 weeks, monthly for 6 months and every 3 months for the next 24 months and underwent a thorough evaluation for tumor recurrence at each visit. However, there was no tumor recurrence in any of the cases.
Discussion
Our study involved 25 eyes of 25 patients with localized OSSN who presented between January 2017 to January 2020 for which they underwent surgical excision followed by 0.04% MMC.
The mean age at presentation was 64 with a range of 47-87 years.8 The patients did not report any recurrence of tumor after a follow-up period of 24 months, thereby indicating a successful outcome.
OSSN has been reported to be the most common malignancy of the conjunctiva and cornea. 1 Primary surgical excision has been the mainstay of treatment of OSSN. Surgical excision facilitates debulking of the tumor and allows an immediate histopathological diagnosis. Surgical excision when used only has a major disadvantage in the form of a high recurrence rate ranging from 15% to 52%. 9 Therefore several adjunctive treatments have come up which help in decreasing tumor recurrence .Chemotherapeutic agent in the form of Mitomycin-C (MMC) is one such adjunctive therapy. MMC is an alkylating agent which is a DNA synthesis inhibitor and causes cell necrosis and apoptosis. 10 Several studies have reported the use of MMC for both primary and recurrent cases of OSSN with successful outcome. 11, 12, 13, 14, 15, 16, 17
MMC has another advantage that it allows treatment of the entire ocular surface including the conjunctival fornices and thus can destroy subclinical disease and prevent the formation of new tumors elsewhere on the ocular surface. Complications with MMC are common as is evident in our study wherein 5 out of 25 patients (20%) reported an allergic response to MMC in the form of mild redness and itching, which resolved after discontinuing treatment. However, none of our patients reported any other serious complications like uveitis, glaucoma, cataract and scleral ulceration 18, as we had ensured complete epithelial healing and the week on-off regimen of MMC application .